Publication Spotlight
AlphaFold 3 can accurately model glycans with proper stereochemistry only when using the BAP+CCD syntax, but manually creating these inputs is tedious and error-prone. To streamline the process, JAAG—a lightweight, user-friendly web tool—automates the conversion of glycans drawn by users into BAP+CCD syntax. This makes it easier for scientists to model glycoproteins and glycan–protein interactions, helping even AlphaFold 3 beginners generate reliable, stereochemically valid structures effortlessly.
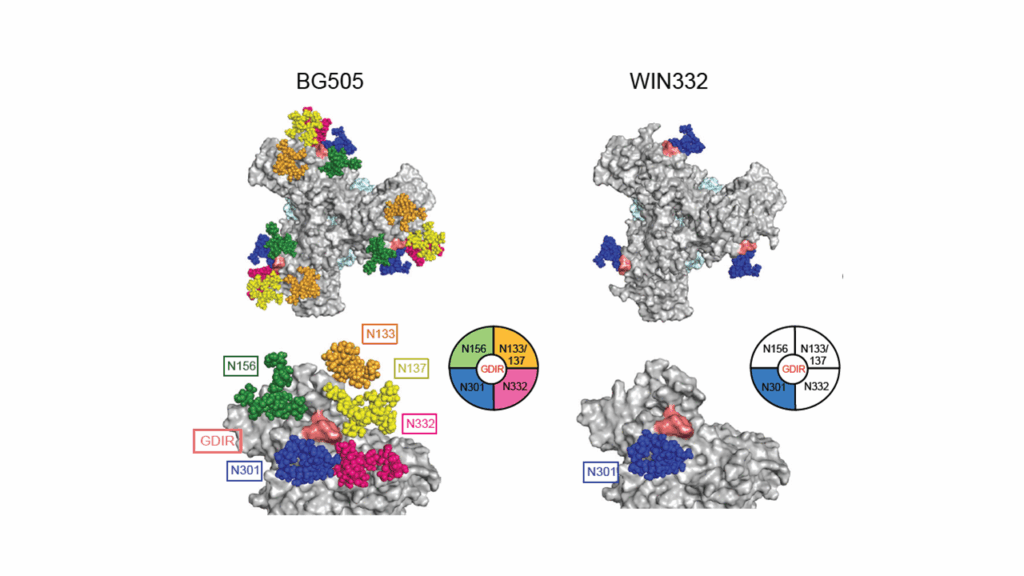
WIN332 binds to precursors of canonical human N332-glycan-dependent (Type-I) V3-glycan bNAbs but also of a first-of-its-class N332-glycan-independent (Type-II) V3-glycan bNAb. WIN332 elicits N332-glycan-independent antibodies with remarkable sequence and binding similarities with the most potent human type-I and type-II V3-glycan bNAbs. Thus, WIN332 is a promising vaccine candidate to streamline V3-glycan bNAb elicitation.
List of All BioF:GREAT Publications
Publication List
JAAG: a JSON input file Assembler for AlphaFold 3 with Glycan integration
Modeling glycans with AlphaFold 3: capabilities, caveats, and limitations
Rapid elicitation of a new class of neutralizing N332-glycan independent V3-glycan antibodies against HIV-1 in nonhuman primates
Comprehensive evaluation of cleavable bioorthogonal probes for site-specific O-GlcNAc proteomics
https://www.mcponline.org/article/S1535-9476(25)00163-X/fulltext
A vascular-associated fibroblastic cell controls pancreatic islet immunity
An updated sulfate transporter phylogeny uncovers a perennial-specific subgroup associated with lignification.
Novel HIV-1 fusion peptide immunogens using glycan-engineered alphavirus-like particles
https://www.nature.com/articles/s41541-025-01288-6
TBR is an RG-I rhamnose O-acetyltransferase required for epidermal cell adhesion in Arabidopsis thaliana
Deciphering the unique autoregulatory mechanisms and substrate specificity of the understudied DCLK3 kinase linked to neurodegenerative diseases
https://www.sciencedirect.com/science/article/pii/S0021925825025165
Human Proteoglycan Linkage Region Glycosyltransferases are Dimeric and Show Unexpected Specificities
https://onlinelibrary.wiley.com/doi/10.1002/anie.202516855